What are biomolecular condensates?
Biomolecular condensates are formed through a process called liquid–liquid phase separation. This is similar to how oil droplets form in water. In cells, certain proteins and RNAs have multiple weak interaction sites. When their concentration or conditions (like salt concentration or temperature) reach a certain threshold, these molecules begin to stick together, separating into a dense, droplet-like phase and a more dilute surrounding phase.
These droplets are not enclosed by membranes, but they behave like liquid compartments concentrating specific molecules to speed up or regulate biochemical reactions. Their formation is often dynamic and reversible, allowing cells to respond quickly to changes.
Summary of the findings:
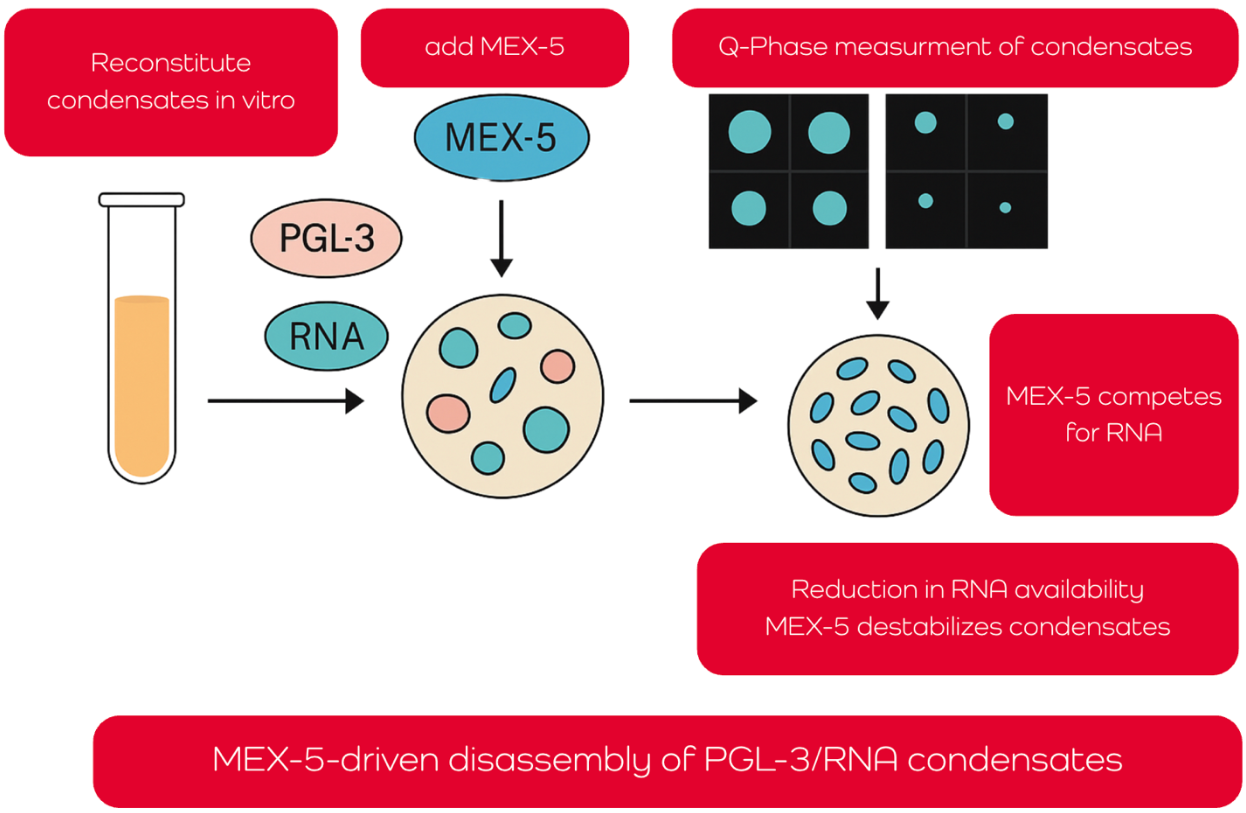
The article examines how the RNA-binding protein MEX-5 regulates the disassembly of biomolecular condensates, particularly PGL-3/RNA droplets that mimic P granules in C. elegans embryos. These membrane-less condensates are essential for germ cell specification and form through liquid–liquid phase separation. Using a fully reconstituted in vitro system, the researchers demonstrate that MEX-5 dissolves preformed condensates by competing for RNA, thereby reducing RNA availability for PGL-3 and shifting the phase boundary required for droplet stability.
The team employed advanced microfluidics and quantitative phase imaging (QPI), using the Q-Phase to map how varying concentrations of RNA, PGL-3, and MEX-5 affect condensate formation. Q-Phase enabled precise, label-free measurement of condensate density and composition, revealing that MEX-5 lowers the free energy needed for phase separation, effectively destabilizing droplets. Mutant MEX-5 lacking its zinc finger domain failed to disassemble condensates, confirming the essential role of this RNA-interacting region.
Together, the findings offer a quantitative, mechanistic framework for how RNA-binding proteins regulate condensate stability. This contributes to a broader understanding of cellular organization, polarity, and development, and demonstrates the power of in vitro condensate reconstitution while using non-invasive imaging technologies. Link to a full article.
Terms used in article
Biomolecular condensates are dynamic, membrane-less compartments formed by proteins and RNA inside cells. They organize molecules to carry out specific functions. Examples include stress granules and P granules. Their formation and dissolution are regulated processes critical to cell function and development.
MEX-5 is an RNA-binding protein in C. elegans embryos. It helps dissolve RNA-protein droplets (P granules) by binding RNA, making it unavailable for other proteins like PGL-3. This controls the formation of these droplets within the cell, influencing cell fate determination toward germ cell development.
PGL-3 is a protein that combines with RNA to form P granules in early embryos. These droplets are essential for germ cell development. PGL-3 is a key factor for the formation and maintenance of these phase-separated structures until they are actively dissolved by factors like MEX-5.
RNA condensates are droplet-like clusters formed by RNA and proteins through weak, reversible interactions. They have no membrane and help compartmentalize biochemical processes inside cells. In this study, they represent simplified P granules built from PGL-3 and poly-rU RNA in a test tube.
P granules are droplet-like structures in C. elegans embryos, made of RNA and proteins like PGL-3. They are formed by phase separation and are crucial for germline specification. Their disassembly in certain cell regions is key to proper embryonic development.
Caenorhabditis elegans is a small roundworm widely used in biology research. Its transparent body and simple development make it ideal for studying how cells divide, specialize, and organize. This model organism was the key to discovering how MEX-5 controls RNA condensates.
How Did Q-Phase Contribute?
- The optical density of the droplets was quantified based on the reduction in transmitted light intensity, indicating their relative concentration and compactness.
- Even slight variations in the amount of protein and RNA levels inside the droplets were detectable across various conditions and time points.
- Measurements performed by Q-Phase elucidated how MEX-5 modulates the balance inside the droplets/condensates, ultimately promoting their disassembly.
To dive into the topic more, read the full article. If you are interested in the subject of biomolecular condensates and their measurement with QPI, you can watch a webinar below.