What is cell mass in the context of imaging?
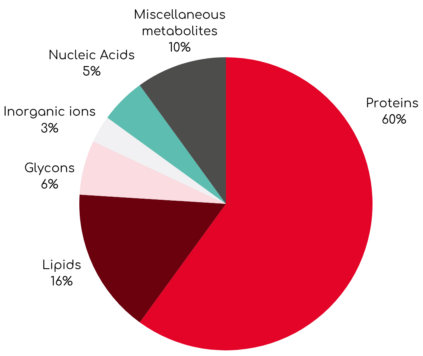
Cellular activities, such as growth, division, and migration, involve the dynamic redistribution of intracellular material. Quantitative Phase Imaging (QPI), the technology behind the Q-Phase system, allows precise measurement of cellular material by detecting the light delay as it passes through a cell. This delay, known as phase shift, is measured and converted to cell dry mass density (pg/μm²) novel, quantitative parameter that indicates the amount of biomaterial the cell contains per unit area. Cell dry mass represents the total amount of non-aqueous components within a cell, including proteins, lipids, nucleic acids, and carbohydrates. Importantly, this parameter serves as a sensitive indicator of a cell’s health and metabolic activity.
Cell dry mass composition
How can cell mass be measured to better understand intracellular mechanics?
In this new study, the authors from CEITEC – Brno University of Technology have introduced an analysis tool called Analytical Image Differencing (AID) to assess the dynamic redistribution of cell mass from QPI data. This concept builds upon a specific digital imaging method used to analyze live cell behavior – Dynamic Phase Differences (DPD). In the DPD method, two subsequent overlapping images of a cell from a time-lapse series are compared. The AID method extends the DPD approach by incorporating new metrics: centroids of regions with increased and decreased cell mass, corresponding position vectors, Mass Transfer Vector (MTV), and the zero-line concept (ZL). ZL marks the boundary between regions of increased and decreased mass, indicating where the values of the cell’s mass have not changed during cell movement. In addition, researchers suggest that the length of the ZL may serve as a new biomarker for distinguishing different types of cell movement.
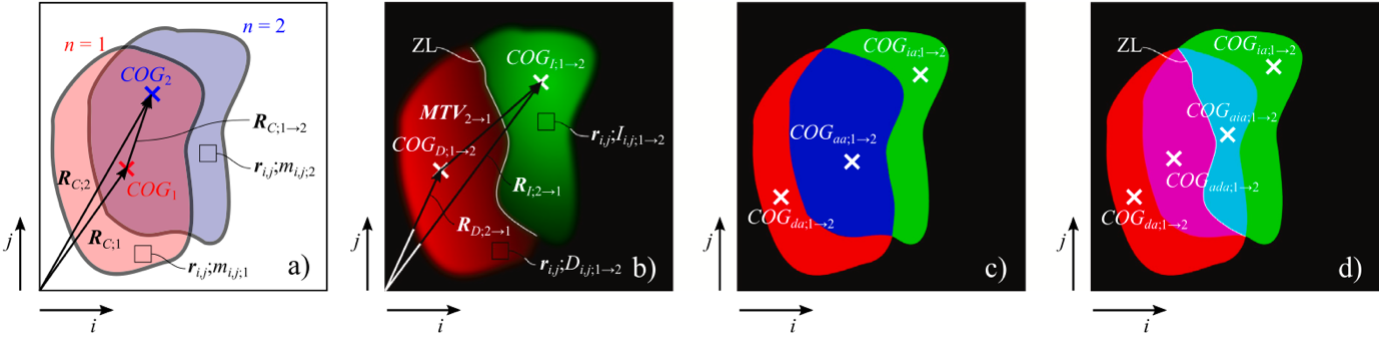
Principle and definition of AID analysis for two consecutive QPI images 𝑛 = 1 … 2: (a) QPI overlay of two subsequent images of the cell, (b) the AID image of the increment (coded in green shades) and decrement (coded in red shades) regions with a white marked zero-line ZL, (c) visualization of increment area (green color), decrement area (red color) and anchor area (blue color), (d) extended anchor visualization – anchor decrement area (purple color) and anchor increment area (turquoise color).
Researchers at CEITEC developed Analytical Image Differencing to analyze dynamic changes in cell mass using QPI data. With the Q-Phase microscope, they quantified intracellular mass flow with high precision and minimal noise. This method enables real-time label-free observation of live cells and introduces new biomarkers like the zero line to better understand cell behavior and migration. Link to the full article!
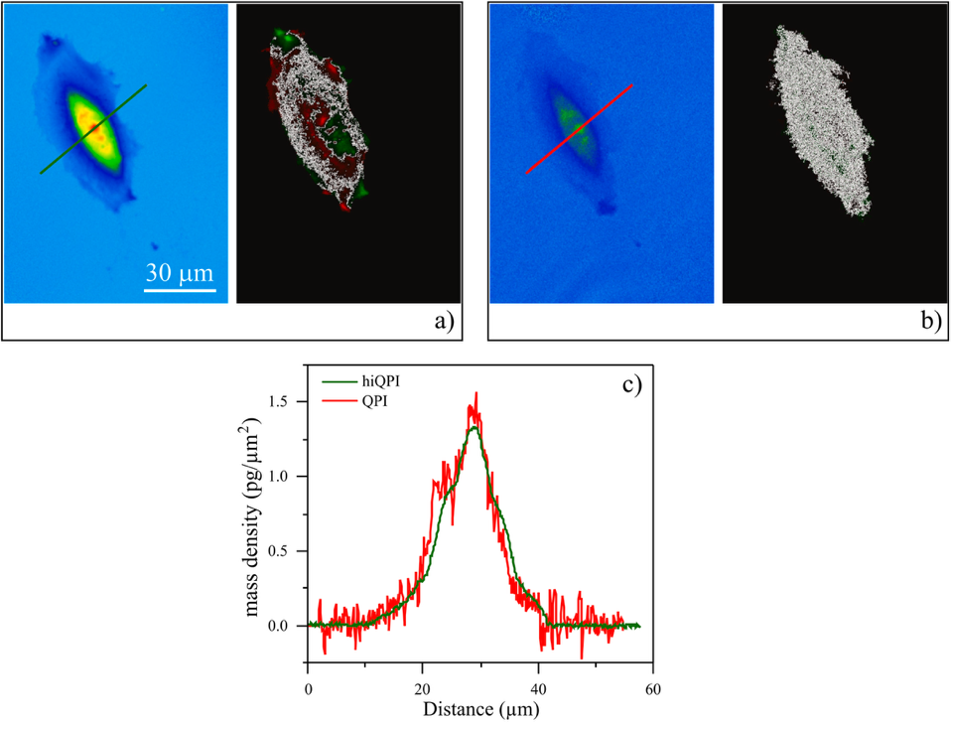
Comparison of hiQPI and coherent-light QPI imaging using a spatially coherent light source. (a) The hiQPI image, along with the corresponding AID image, was computed for a time difference of 𝛥𝑡 = 180 s. The image was acquired with an illumination aperture of 𝑁𝐴 = 0.30. (b) The coherent-light QPI image along with the corresponding AID image, computed for a time difference of 𝛥𝑡 = 180 s. The image was acquired with an illumination aperture of 𝑁𝐴 = 0.01. (c) Cross-sections of hiQPI (green color) and coherent-light QPI (red color) images for comparison of noise introduced by the degree of spatial coherence of the light source.
Highlights:
- Low-noise, high-precision cell mass measurements
- Simultaneous graphical and numerical output
- High-resolution tracking of mass dynamics
- High-quality input data for AID analysis
The terms used in the article
A method that uses an incoherent broad-spectrum light source to achieve higher lateral and axial resolution with lower noise than traditional coherent light QPI. This technique enables precise, label-free imaging of live cells and accurate measurement of their mass redistribution.
An image analysis method that subtracts two consecutive QPI images to detect regions of mass increase and decrease, as well as the zero-line separating them.
Optical contrast between dense and dilute phases, linked to solute concentration.
A newly introduced parameter representing the boundary between areas of increased and decreased mass, serving as a potential biomarker of cell motility type.
A vector connecting the centroids of mass increase and decrease areas, indicating the primary direction of intracellular mass flow.
How did Q-Phase contribute?
In order to integrate new AID metrics into the analysis of cell-mass transfer, it was essential to measure cell mass changes with extremely high precision. These data were acquired using the Q-Phase microscope, a system utilizing the hiQPI (Holographic Incoherent-Light-Source Quantitative Phase Imaging) principle. Unlike conventional coherent-light QPI systems that rely on lasers, the Q-Phase employs an incoherent light source, which helps minimize interference noise on output images.
The study further compared hiQPI with coherent-light QPI imaging. It showed that input images with higher noise levels reduce the accuracy of AID measurements and can lead to incorrect ZL detection. The comparison shown in Figure 2 demonstrates that coherent illumination results in significant noise and elongates the ZL in the AID image, whereas the Q-Phase’s incoherent illumination provides more precise mass measurements and consequently more reliable AID outputs. Overall, the system simultaneously provides graphical and numerical data, ensuring both qualitative visualization and quantitative evaluation.