Definition
Cell proliferation is an increase in the number of cells due to cellular growth and cell division. When cells increase their mass and volume without increasing in number this is cell growth. In contrast cell division is the formation of two daughter cells from a parent cell. Thus, cell proliferation is a composite of two cell behaviors—growth and division. Cell proliferation represents a balance between the loss of cells due to death or differentiation and an increase in cell number due to cell division. For multicellular organisms, cell proliferation is important for the growth of the organisms and wound healing. In contrast, uncontrolled cell proliferation is a hallmark of cancer. A list of the latest reviews and research on cell proliferation can be found here and here.
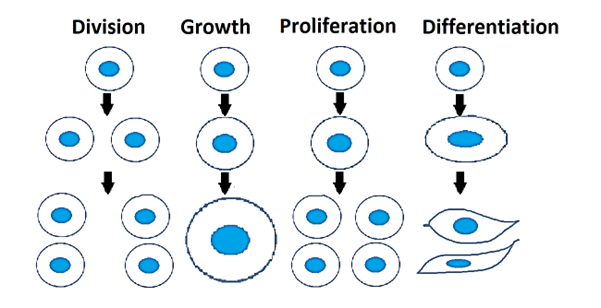
Figure 1: Cell division, growth, proliferation, and differentiation. Filled blue ovals represent nucleus.
Cell proliferation and differentiation
Cell proliferation is increasing the number of cells whereas cell differentiation is acquiring a special function and/or shape (morphology) by a less specialized cell. Differentiation is often achieved in stages and terminal differentiation is commonly associated with permanent exit from the cell cycle. Thus, fully differentiated cells cannot divide further. During normal development, there is an inverse relationship between proliferation and differentiation. This is important for maintaining tissue homeostasis and cell replacement. Figure 2 depicts differentiation where single-celled myoblasts differentiate into myotubes (green) containing multiple nuclei.
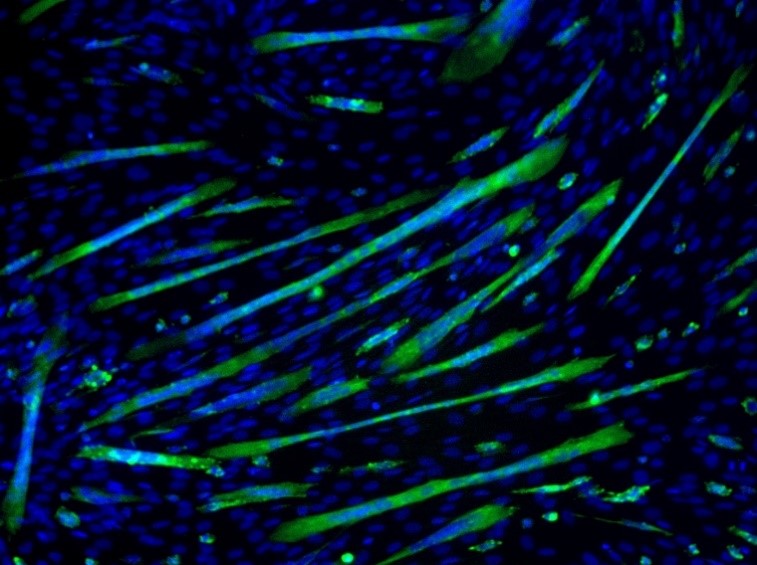
Figure 2: Cell differentiation: Myoblast cells forming multinuclear myotubes. Image sourced from CRBS cell mage library
Examples of cell proliferation
Examples of proliferating cells in adult humans include skin fibroblasts, intestinal epithelial cells, and liver regeneration following surgery or massive cell loss. The formation of different blood cells encompasses both proliferation and differentiation of hematopoietic stem cells. A detailed discussion on proliferation can be found here.
Measuring cell proliferation
Proliferating cells have active metabolism and DNA synthesis preceding cell division. These features are exploited to obtain rates of cell proliferation. The following table lists the reagents that are commonly used to measure cell proliferation.
Table 1: The nucleotide analogues used to detect cell proliferation through DNA replication.
| Reagent |
Principle |
Method of assay |
Advantage |
Disadvantages |
|---|---|---|---|---|
| BrdU (5-bromo-2′-deoxy-uridine assay) |
|
BrDU incorporated cells are stained with anti BrDU antibody |
Applicable to cells and tissue |
Requires additional antibody and DNA denaturation |
| EdU (5-ethynyl-2´-deoxyuridine) |
Applicable to cells and tissue |
|
Table 2: Nuclear proteins used to measure cell proliferation.
| Reagent |
Principle |
Method of assay |
Advantage |
Disadvantage |
|---|---|---|---|---|
| Ki-67 |
Present in S, G2, and M phase cells |
Immunohistochemistry and flow cytometry-based detection using monoclonal antibody against the Ki-67 protein |
Useful for measuring proliferation of tumours |
End point assay requires tissue fixation |
| Phosphorylated histone 3 (PH3) |
Present in G2 and M phase cells, marking condensed chromatin prior to chromosomal segregation |
Immunohistochemistry using specific molecular antibodies |
Proliferating tumours, more specific |
End point assay |
Table 3 Reagents measuring cell proliferation through metabolic activity
| Reagent |
Principle |
Method of Assay |
Advantages |
Disadvantage |
|---|---|---|---|---|
| MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
|
|
Easy to perform
|
End point assay
|
| XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)
|
||||
| MTS |
||||
| WTS |
||||
| Alamar blue |
Soluble in medium |
Positive reaction requires presence of at least 100 cells per dish |
Table 4 Measuring cell proliferation dynamically
| Reagent |
Principle |
Method of Assay |
Advantages |
Disadvantages |
|---|---|---|---|---|
| Carboxy fluorescein diacetate succinimidyl ester (CFSE |
CFSE by itself is non fluorescent, once it is inside viable cells it is cleaved by cytoplasmic esterase enzyme to generate fluorescent dyes which react with amine groups of protein forming stable conjugates. Upon division the fluorescence is halved in daughter cells and thus proliferation can be followed through generations. |
Cells are incubated in PBS containing CFSE and then washed to remove lingering extracellular stain. Cells are then followed either by flow cytometry or in microplate reader |
Cell viability is not affected, dynamic assay, can follow cells through multiple generation. |
Cells can be followed till a specific number of generations beyond which background noise affects detection. |
Cell proliferation rate calculation
Cell proliferation is generally measured by estimating the increase in the number of cells (cell count) over time starting with a specific number of parental cells. The cell count is obtained after staining the cells (with fluorescent markers, antibodies, or colorimetric reagents) to select dividing or metabolically active cells. Cell counts at different time points are utilized to calculate proliferation. The proliferation rate is expressed as a proliferation index. Different software utilize different formulae to calculate this proliferation index. For example, proliferation measurements using tracking dyes often use the following formula to obtain the proliferative index:
![]()
Cell proliferation assay
Features such as DNA replication and high metabolic activity are often used as endpoint measurements of proliferation, meaning the cells used for measurement once cannot be reused for other experiments. This is due to requirement of fixation of the cells/tissue or prolonged staining for carrying out the measurement. Even techniques employing live cells using fluorescent markers may be restricted by the number of generations that can be followed with the labelling agent. Depending on the choice of reagents used, some information regarding the dynamic progression of cell proliferation over time may be lost. Similarly, to test the effects of chemicals/drugs that modulate proliferation, multiple parallel cultures might be necessary if such end point measurements are done throughout different time points. Measuring proliferation of live cells non-invasively over time takes care of these issues and provides the missing temporal information. Moreover, cells in a population might exist as an ensemble with different proliferating abilities across time and this information will only be captured if the proliferation of live cells is tracked across different timepoints. Adapting non-invasive proliferation measurements in a multi-well format allows simultaneous comparison of different drugs.
Bibliography
Munson M. An improved technique for calculating relative response in cellular proliferation experiments. Cytometry Part A. 2010;77A(10):909-910. doi:10.1002/cyto.a.20935
Roederer M. Interpretation of cellular proliferation data: Avoid the panglossian. Cytometry Part A. 2011;79A(2):95-101. doi:10.1002/cyto.a.21010